Cellular NAD+ Levels
Life-sustaining NAD+ depletes with age but can now be replenished to maintain DNA repair capacity and cell vitality.
Nicotinamide adenine dinucleotide (NAD+) is a vital molecule inhabiting all living cells. NAD+ is essential in regulating energy metabolism and provides the substrate necessary to fuel enzymes that modulate key processes like DNA repair and cell survival. However, due to increased consumption and reduced synthesis, cellular NAD+ levels gradually decline with age. This loss of NAD+ leads to inefficient energy production that promotes mitochondrial dysfunction. Furthermore, without sufficient NAD+, cells become less resilient to stress and damage, leading to senescence (biological obsolescence). The consequences of senescence and mitochondrial dysfunction culminate in organ dysfunction, ultimately manifesting in age-related conditions.
NAD+ Fuels Sirtuins
Sirtuins are a family of enzymes that modulate protective processes essential for cell survival, including DNA repair. These survival enzymes consume NAD+ to fuel their activity, so when cellular NAD+ levels drop, sirtuin activity diminishes. Thus, the decline in NAD+ that occurs with aging is intricately linked to reduced sirtuin activity. Increasing sirtuin activity has been shown to ameliorate age-related health conditions and extend the lifespan of multiple organisms, from yeast to mice.
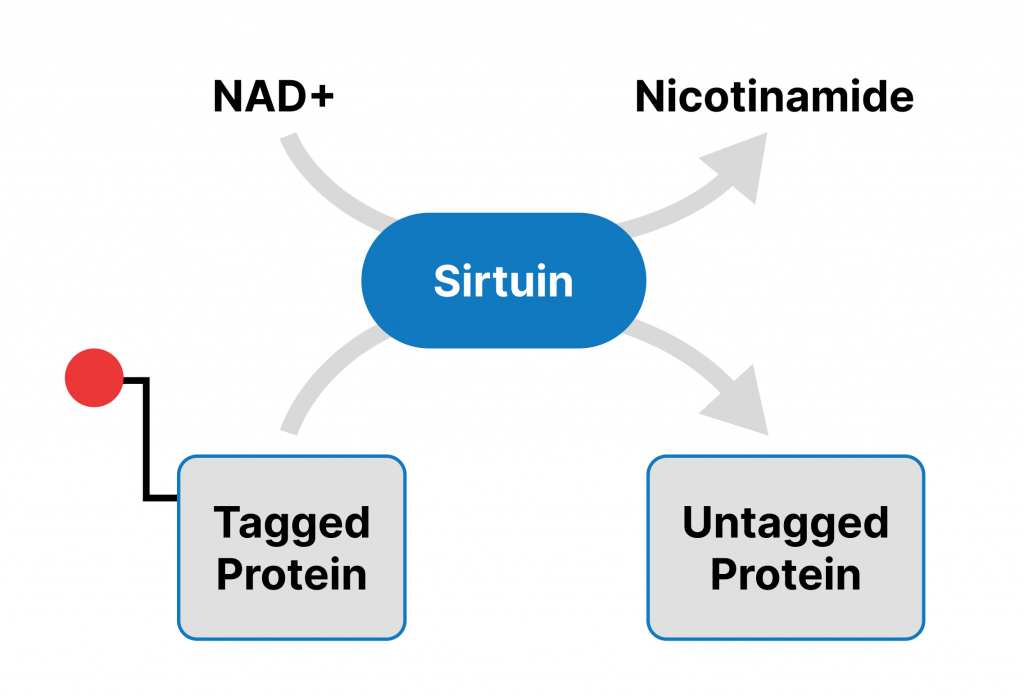
DNA Damage and Cellular Senescence
Our DNA is prone to damage, which can be triggered by factors such as ultraviolet (UV) radiation, environmental pollutants, inflammation, and DNA replication errors. To limit the damage inflicted upon DNA, our cells activate sirtuins to modulate DNA repair processes. Without sufficient levels of NAD+ and sirtuin activity, DNA damage accumulates and eventually triggers cellular senescence. Senescence is when a cell becomes dormant and begins secreting molecules involved in degrading organ tissue and promoting inflammation. Senescent cells are usually cleared out by the immune system, but with age, senescent cells accumulate and can contribute to the dysfunction and deterioration of the organs and tissues they inhabit. Cellular senescence is considered one of the key underlying features of aging.
Mitochondria and Oxidative Stress
Sirtuins modulate a diverse array of cellular processes by modifying key proteins, including proteins involved in mitochondrial maintenance. Mitochondria produce most of our cell’s energy, ATP, which sustains cell function and survival. Mitochondria are in constant need of maintenance to maximize their production of ATP. Without this maintenance, mitochondria become dysfunctional and begin to produce excessive levels of reactive oxygen species (ROS). At high levels, ROS cause damage to cells — a phenomenon known as oxidative stress. Thus, by modulating mitochondrial maintenance, sirtuins help cells function optimally by assuring sufficient levels of ATP and limiting oxidative stress.
Effects of Restoring NAD+
Numerous animal studies have revealed that NAD+ levels decrease with age in many tissues, including the liver, muscle, and brain. In humans, NAD+ levels have been reported to decrease in the skin, brain, liver, and blood-plasma. Replenishing NAD+ leads to increased insulin sensitivity, improved sleep and physical performance, enhanced exercise endurance, improved skin health, increased walking speed, and increased strength in middle-aged and older adults. Additionally, increasing NAD+ prolongs the lifespan of mice, delays muscle degeneration, inhibits bone deterioration, and enhances tumor suppression. Also in rodents, raising NAD+ levels delays cardiovascular aging and prevents neurodegeneration while improving memory.
How to Replenish NAD+
Insufficient concentrations of cellular NAD+ are associated with mitochondrial dysfunction, oxidative stress, inflammation, DNA damage, cellular senescence, and the eventual metabolic collapse associated with age-related health conditions and mortality. Therefore, finding ways to replenish NAD+ becomes key to healthspan extension.
NAD+ Precursors
NAD+ precursors are molecules used by cells to synthesize NAD+. Some NAD+ precursors exists in small quantities in food, including B3 vitamins. There are three known B3 vitamin NAD+ precursors, namely nicotinamide, nicotinic acid, and nicotinamide riboside (NR). Tryptophan, an amino acid, is also an NAD+ precursor, although it is nine (metabolic) steps away from becoming NAD+ once it gets into the cell. Accordingly, nicotinamide and nicotinic acid are three steps away, and NR is two steps away from being synthesized into NAD+. The most recently discovered NAD+ precursor is nicotinamide mononucleotide (NMN), which is one step away from becoming NAD+.
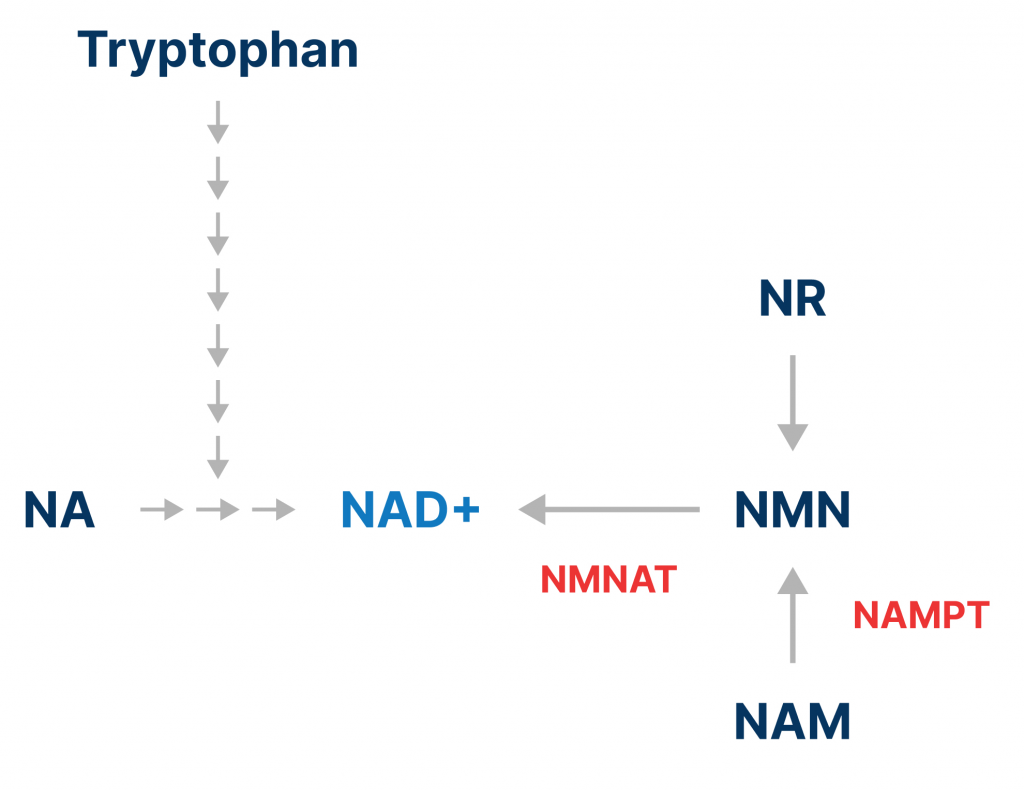
NAD+ precursors are used to raise cellular NAD+ levels because NAD+ itself cannot readily enter cells. Furthermore, orally administered NAD+ is quickly metabolized, preventing it from reaching the bloodstream. The most widely studied NAD+ precursors are NR and NMN, which have been shown to increase circulating NAD+ in rodents and humans.
Inhibiting NAD+ Breakdown and Activating NAD+ Synthesis
NAD+ levels decline with age due to an increase in NAD+ consumption (breakdown) and a decrease in NAD+ synthesis. One of the primary NAD+ consumers is an immune cell enzyme called CD38, which increases in activity with age. Studies have linked CD38 hyperactivation to organ and tissue dysfunction, prompting the use of CD38 inhibitors to restore NAD+ levels and help prevent age-related health conditions.
Another consumer of NAD+ is PARP1, an enzyme involved in DNA repair. While studies have shown that inhibiting PARP1 effectively increases NAD+, limiting PARP1 activity could slow down DNA repair, potentially accelerating the development of age-related conditions. PARP1 also competes with sirtuins for NAD+ and is thought to be one of the reasons sirtuin activity declines with age.
While the consumption of NAD+ increases with age by enzymes like CD38 and PARP1, at the same time, the synthesis of NAD+ decreases. This is because one of the enzymes responsible for NAD+ synthesis called NAMPT declines with age. NAMPT converts nicotinamide into NMN. One way to increase the synthesis of NAD+ is to increase the activity of NAMPT. NMNAT, the enzyme responsible for converting NMN to NAD+ can also be activated to increase NAD+ synthesis.